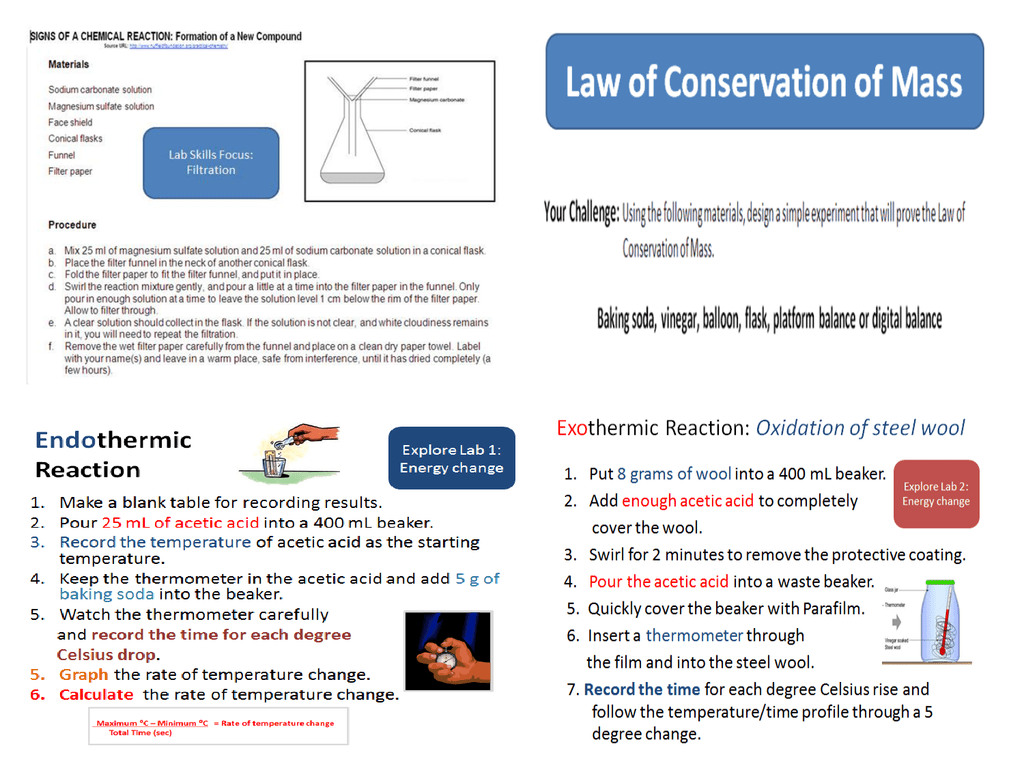
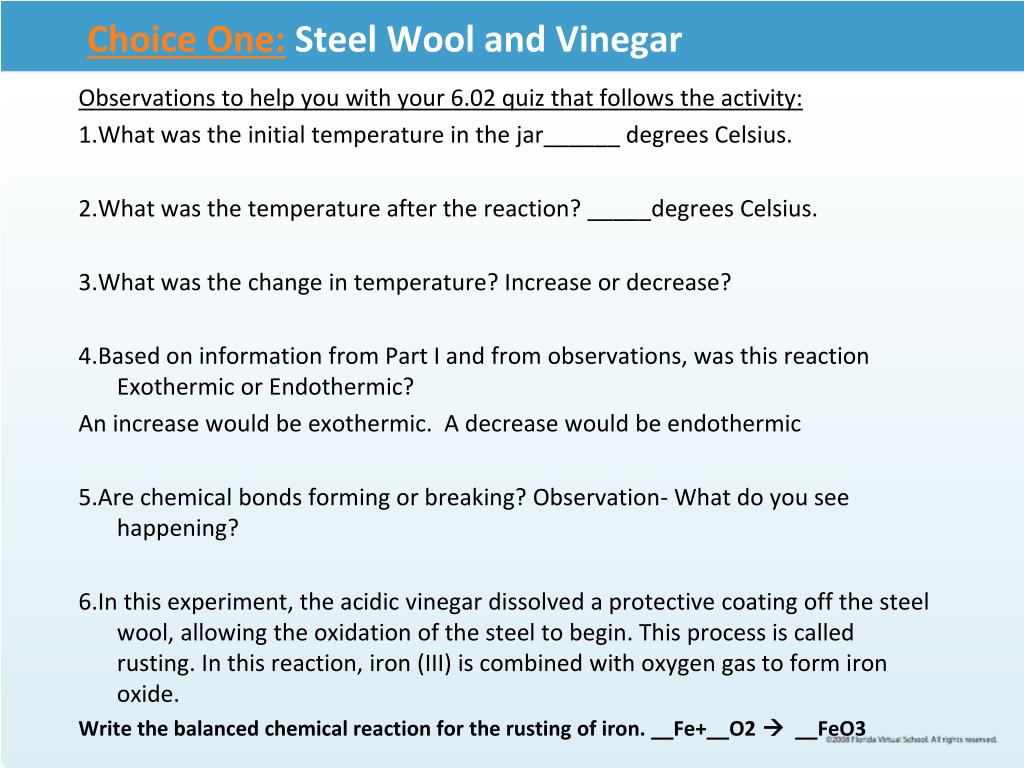
Vinegar And Steel Wool Chemical Reaction
Rusting or oxidation is a chemical reaction between iron and oxygen this chemical reaction creates heat energy which increases the temperature inside the beaker.

Vinegar and steel wool chemical reaction. The thermal energy given off during this chemical reaction causes the mercury in the thermometer to expand and rise up the column of the thermometer tube. Who knew that steel wool and vinegar creates heat. Music aurora borealis from youtubes. Steel wool is not soluble in water neither can it absorb water but can get wet so if you take the steel wool out of the water the wet due to capillary action and surface tension steel wool will.
When you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. When iron is exposed to oxygen rust forms. Not only does the vinegar remove the protective coating on the steel wool but once the coating is off its acidity aids in oxidation rust of the iron in the steel. This chemical reaction creates heat energy which increases the temperature inside the beaker.
Steel wool contains iron. Steel wool and vinegar reaction. Rusting or oxidation is a chemical reaction between iron and oxygen. Rust is a product of a chemical reaction between iron and oxygen.
Both plain steel wool and hydrogen peroxide are available at most supermarkets. At the end of this episode you will be able to demonstrate what happens when you place steel wool into vinegar explain why the steel wool rusts so quickly and explain what an oxidation reaction is. So easy and safe to try for yourself. Peroxide is the short term for 3 household hydrogen peroxide.
When you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Steel wool reacts vigorously with hydrogen peroxide but only under. Steel wool has a protective coating that keeps oxygen from coming into contact with the iron.