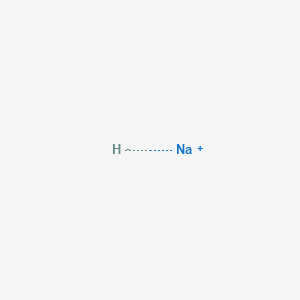
How To Remove Mineral Oil From Sodium Hydride
View information documentation regarding sodium hydride including cas msds more.

How to remove mineral oil from sodium hydride. Using 60 sodium hydride dispersed on mineral oil is much safer than using pure sodium hydride. Spontaneous ignition in air can occur. Swirl with hexaneheptane let it settle pipette off the clear solvent. Sigma aldrich offers a number of sodium hydride products.
Repeat then add your reaction solvent. Examples include hexane heptane diethyl ether dibutyl ether and toluene. In contact with water it releases flammable gas hydrogen which may ignite spontaneously. Sodium hydride is a severe irritant to skin and eyes.
Such a dispersion is safer to handle and weigh than pure nah. Viscosity 75 85 sus at 1000f. Ive never seen it packaged dry and i dont want to. Sodium hydride nah is strongly water reactive.
Form microcrystalline dispersion of gray powder in mineral oil. Such dispersion is safer to handle and weigh than pure nah. Sodium hydride is sold by many chemical suppliers usually as a mixture of 60 sodium hydride ww in mineral oil. Sodium hydride nah reacts violently with water liberating hydrogen gas.
60 3 diluent mineral oil. Place your weighed sodium hydride dispersion in the reaction flask. Most of the time it is not necessary to remove it. It ignites much more easily than the sodium compound to the point that people even manage to start fires with the oil soaked item.
The mineral oil is inert and will not interfere with your reaction and runs at the top of a tlccolumn. I used both the nah and kh suspensions a couple of times during my phd for some synthetic preparations and it is fairly straightforward the main thing to keep in mind is that they can react extremely vigorously with water releasing heat and a. The related potassium hydride is invariably sold in a hard to handle suspension thats mostly oil. All reactions should be done under an inert atmosphere.
Sodium hydride is sold as a mixture of 60 sodium hydride ww in mineral oil. At least the flames are prettier.